
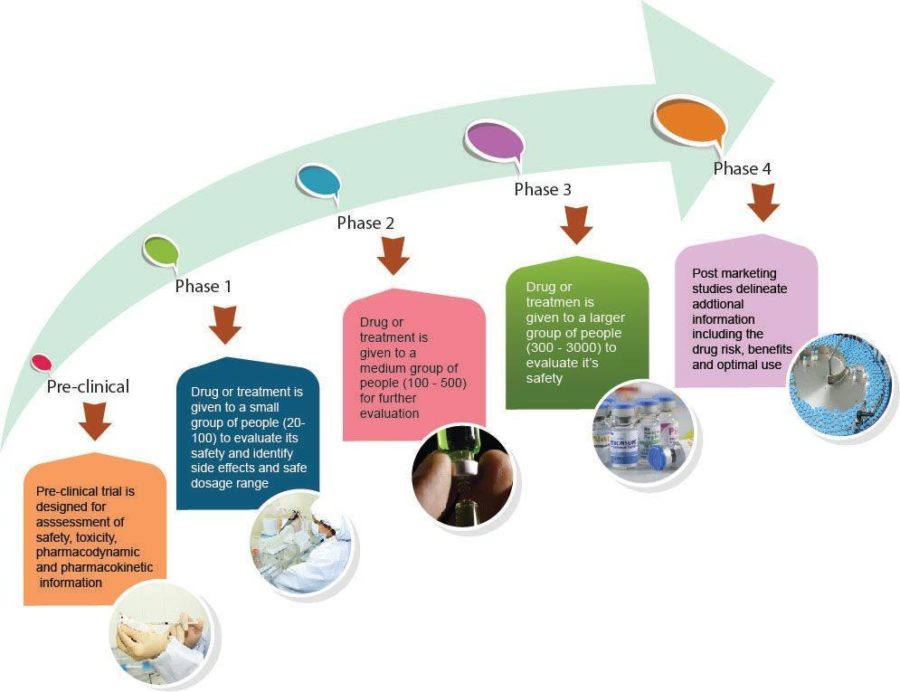
CLINICAL STUDIES TRIAL
to support and verify the compilation of the Clinical Trial Application form of clinical studies Clinical trials and research studies help us understand childhood diseases and find better ways to prevent and treat them. Island Healths Clinical Trials Unit supports researchers to expand the number of studies and treatment options we offer to patients and their families.to act as a contact for communications with the portal of the National Observatory on Clinical Trials of Medicines (OsSC).to agree with the study team upon the timing of submission.to support the study team in defining and drafting the documentation produced from a regulatory point of view, verifying its adequacy.to classify the necessary documentation and the approval process to be followed based on the type of study.It deals with support/consultancy for the activation of clinical trials and clinical research projects promoted by the institute. NZCR provides state of the art world class research units and the expertise to conduct complex early phase clinical research in healthy participant and. The activity of Regulatory Affairs for Clinical Studies aims to support, advise and assist the Laboratories that deal with clinical studies regarding the regulatory aspects during start-up activities and throughout the course of the study. consulting on legislation and requirements for conduct clinical trials.control of the application of procedures and adherence to standardized working methods.Each study answers scientific questions and tries to find. We are grateful to the patients, healthy volunteers, hospitals and clinics that participate in the clinical trials for testing our potential therapies.


To achieve this goal, the following activities are carried out: It also guarantees the maintenance of the health accreditation requirements of the Clinical Research Center for Rare Diseases Aldo and Cele Dacc, according. The Quality Assurance for Clinical Studies activity therefore aims to develop and maintain a Quality Assurance System, that ensures adherence to the required standards, during the execution of the Institute's clinical studies, in accordance with current regulations. In accordance with the Guideline for Good Clinical Practice - GCP, E6 (R2), Step 5 1 December 2016 EMA / CHMP / ICH / 135/1995 "Committee for Human Medicinal Products") " who promotes clinical studies is responsible for implementing and maintaining quality assurance and quality control systems with written SOPs to ensure that trials are conducted and data are generated, documented (recorded), and reported in compliance with the protocol, GCP, and the applicable regulatory requirement(s) (5.1.1) ". The main scope of the Quality Assurance and Regulatory Affairs for Clinical Studies activity of the Mario Negri Institute of Pharmacological Research IRCCS is to ensure that all clinical studies are conducted according to current regulations.


 0 kommentar(er)
0 kommentar(er)
